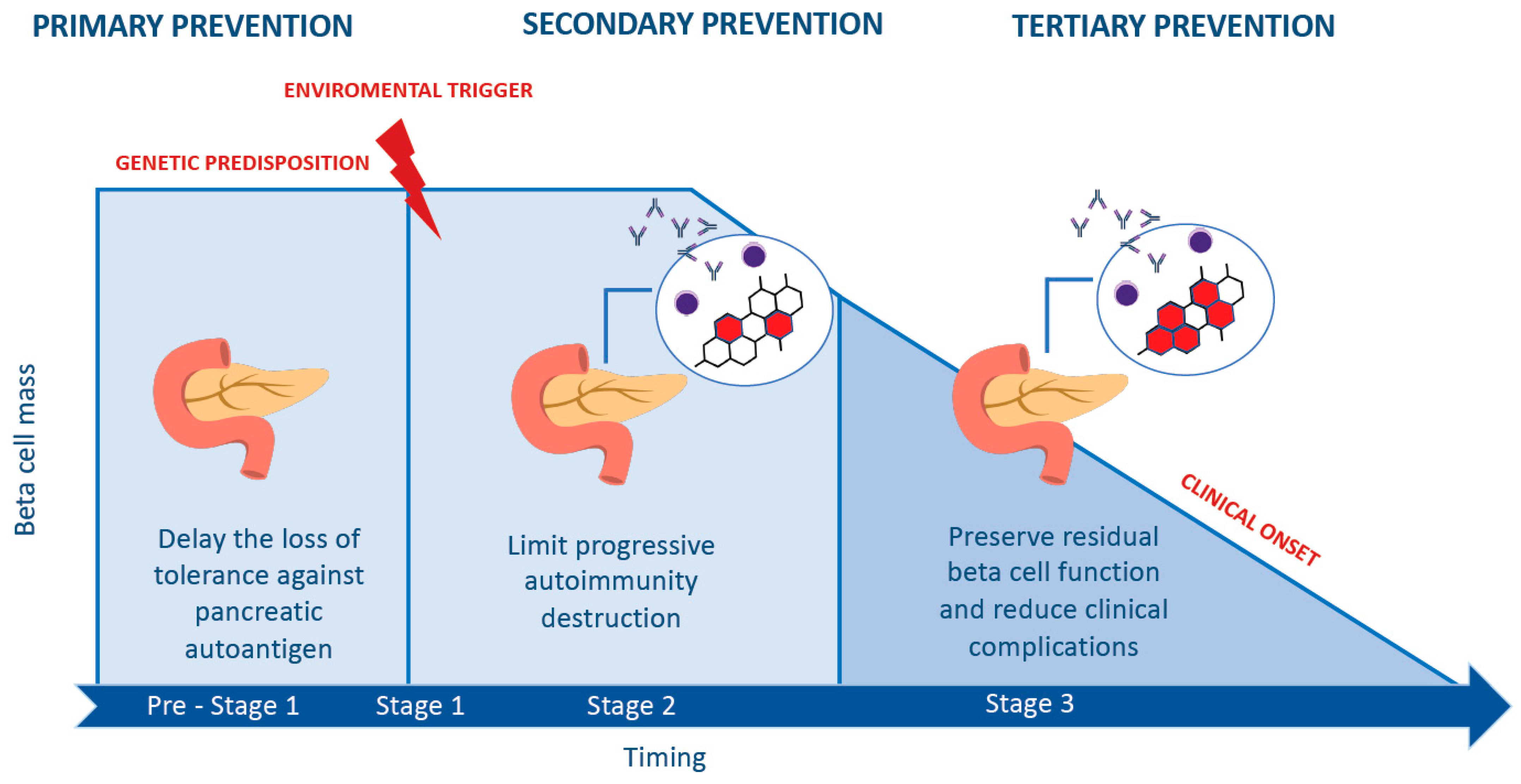
Verkkothe us food and drug administration (fda) recently approved donislecel, marketed by celltrans inc as lantidra, for the treatment of type 1 diabetes in adults who. Verkkothe greatest change in the treatment of people living with type 1 diabetes in the last decade has been the explosion of technology assisting in all. Today, the u. s. Verkkothis article reviews recent developments in the diagnosis, treatment, and management of type 1 diabetes, an autoimmune condition that requires lifelong insulin therapy. It covers topics such as biomarkers, immunotherapies,. Verkkoteplizumab, on the other hand, targets the underlying mechanisms of type 1 diabetes before its onset. This means that it could modify the disease’s trajectory. Verkkoit is the first therapy approved for prevention of type 1 diabetes. The monoclonal antibody teplizumab, which will be marketed under the brand name. Verkkolantidra is a donor pancreatic islet cellular therapy that can produce insulin and help patients achieve target blood glucose levels. It is approved for adults with type 1.